|
|
| RESEARCH ACTIVITIES |
 |
| Singlet Oxygen Generation by Energy Transfer from Silicon Nanocrystals |
|
P59. Toshihiro Nakamura, T. Ogawa, T. Kubota, S. Adachi, and Minoru Fujii,
"Resonant Energy Transfer from Porous Silicon to Iodine Molecules ,”
ECS Transactions, Vol. 16, No. 3, pp. 267-276 (2008).
(Proceedings of ECS 214th meeting,
Honolulu, Hawai, October 12 - 17 (2008). |
| The electronic excitation energy transfer between excitons in porous silicon and iodine molecules in an organic solution is studied. From the time-resolved photoluminescence the rate of the energy transfer is increased with approaching a wavelength region where the photoluminescence spectrum of porous silicon overlaps the absorption spectrum of iodine molecules, and with increasing the radiative recombination rate of porous silicon. We show that the dependence of the rate is well explained by Förster type dipole-dipole interaction mechanism in which the diffusion of the assemblies and molecules is taken into consideration. Furthermore, it is found that the efficiency of the energy transfer strongly depends on the emission wavelength at low iodine concentration. |
|
|
P56. Hirokazu Fumon, Masato Nojiri, Minoru Fujii1, Shinji Hayashi, and
Kensuke Akamatsu
“Sensitized Generation of Singlet Oxygen by Allylamine-Terminated Hydrophilic
Porous Si ,”
Transactions of the Materials Research Society of Japan, Vol. 33, No. 1, pp. 165-167 (2008).
(Proceedings of 18th Materials Research Society of Japan Academic Symposium, Nihon University, Tokyo, December 9 (2007))
|
| We studied the sensitized generation of singlet oxygen by surface-modified hydrophilic porous Si. Porous Si consisting of a network of Si nanocrystals acts as an efficient photosensitizer for the generation of exited state molecular oxygen called singlet oxygen. Singlet oxygen is widely used in chemistry and biology. One of the possible applications of it is photodynamic therapy of cancer (PDT). However, as-prepared porous Si is hydrophobic and thus cannot be used as singlet oxygen photosensitizer in aqueous solution. Therefore, in this work, we modified the surface of porous Si by organic molecules (allylamine). We found that allylamine-terminated hydrophilic porous Si holds the photosensitization ability, although the efficiency of singlet oxygen generation is reduced by the modification. |
|
|
P52. Minoru Fujii, Naoki Nishimura, H. Fumon, Shinji Hayashi, Kensuke Akamatsu,
T. Tsuruoka, M. Shimada, H. Katayama, Dmitri Kovalev, and Bernhard Goller,
“Photosensitization of Oxygen Molecules by Surface-modified Hydrophilic Porous Si,”
European Physical Journal D, Vol. 43, No. pp. 193-196 (2007).
(Published online 24 May 2007)
(Proceeding of ISSPIC 13, July 23-28, 2006, Göteborg (Sweden))
|
| Hydrophilic porous Si is prepared by surface modification with polyethylene
oxide (PEO) molecules. The surface modification is confirmed by infrared
absorption spectroscopy and photoluminescence spectroscopy. The effect
of surface modification on the efficiency of photosensitization of oxygen
molecules, i.e., the efficiency of singlet oxygen generation, is studied.
The PEO-terminated hydrophilic porous Si is shown to hold the photosensitization
ability although the efficiency is reduced by the modification. |
|
 |
89. Minoru Fujii, Naoki Nishimura, Hirokazu Fumon, Shinji Hayashi, Dmitry Kovalev, Bernhard Goller, and Joachim Diener,
"Dynamics of Photosensitized Formation of Singlet Oxygen by Porous Silicon in Aqueous Solution,”
Journal of Applied Physics, Vol. 100, Issue 12, pp. 124302-1-5, December (2006). |
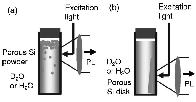
| Generation of singlet oxygen due to energy transfer from photoexcited silicon
nanocrystals in D2O is demonstrated. It is shown that the singlet oxygen
generation efficiency, i.e., the intensity of near-infrared emission from
singlet oxygen gradually decreases when Si nanocrystals are continuously
irradiated in O2-saturated D2O. The mechanism of the photodegradation of
the photosensitizing efficiency is studied using photoluminescence and
infrared absorption techniques. Experimental results suggest that the interaction
of photogenerated singlet oxygen with the hydrogen-terminated surface of
silicon nanocrystals results in photo-oxidation of silicon nanocrystals,
and the surface oxides reduce the photosensitizing efficiency. It is also
demonstrated that photo-oxidation of porous silicon in O2-saturated water
results in a strong enhancement of the photoluminescence quantum yield
of porous Si. |
|
|
74. Minoru Fujii, Dmitri Kovalev, Bernhard Goller, Shingo Minobe, Shinji Hayashi, and Victor Yu. Timoshenko,
"Time-resolved Photoluminescence Studies of the Energy Transfer from Excitons Confined in Si Nanocrystals to Oxygen Molecules,”
Physical Review B, Vol. 72, 165321-1-8, October (2005). |
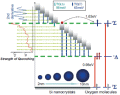
| The formation of singlet oxygen due to the energy transfer from excitons
confined in silicon nanocrystals to oxygen molecules is studied using time-resolved
photoluminescence spectroscopy. The process of the excitation of oxygen
molecules from the ground triplet state to the second excited singlet state
is studied at low temperatures, where oxygen molecules are physisorbed
on the surface of silicon nanocrystals and at room temperature in gaseous
oxygen ambient and in oxygen-saturated water. The low temperature measurements
reveal that the energy transfer time is the shortest for the resonant energy
transfer. The involvement of one energy-conserving transversal optical
phonon results in about 40% increase of the energy transfer time. The excitation
rate of oxygen dimers is found to be similar to that measured for oxygen
molecules. At room temperature, the time of the energy transfer to oxygen
molecules is about 17 μs. The photosensitizing efficiency of silicon nanocrystals
at room temperature is found to be as high as 80% for gaseous oxygen ambient
and for oxygen-saturated water. |
|

 |
73. Dmitri Kovalev, and Minoru Fujii,
"Silicon Nanocrystals: Photosensitizers for Oxygen Molecules,”
Advanced Materials (Review paper), Vol. 17, Issue 21, pp. 2531-2544, November (2005).
|
| Molecular oxygen plays an important role in many of the chemical reactions
involved in the synthesis of biological life. In this review, we explore
the interaction between O2 and silicon nanocrystals, which can be employed
in the photosynthesis of singlet oxygen. We demonstrate that nanoscale
Si has entirely new properties owing to morphological and quantum size
effects, i.e., large accessible surface areas and excitons of variable
energies and with well-defined spin structures. These features result in
new emerging functionality for nanoscale silicon: it is a very efficient
spin-flip activator of O2, and therefore, a chemically and biologically
active material. This whole effect is based on energy transfer from long-lived
electronic excitations confined in Si nanocrystals to surrounding O2 via
the exchange of single electrons of opposite spin, thus enabling the spin-flip
activation of O2. Further, we discuss the implications of these findings
for physics, chemistry, biology, and medicine. |
|
|
68. Dmitri Kovalev, Egon Gross, Victor Yu. Timoshenko, and Minoru Fujii,
"Photodegradation of Porous Silicon Induced by Photogenerated Singlet Oxygen Molecules,”
Applied Physics Letters, Vol. 85, pp. 3590-3592 (2004). |
| We report on the mechanism of photodegradation of porous silicon luminescence in ambient containing molecular oxygen. Energy transfer from excitons confined in silicon nanocrystallites to molecular oxygen results in the generation of highly chemically reactive singlet oxygen molecules. The subsequent interaction of singlet oxygen molecules with the hydrogenated surface of silicon nanocrystals results in its photooxidation and the creation of additional nonradiative defects, i.e., the luminescence fatigue effect. |
|
|
66. Minoru Fujii, Shingo Minobe, Motofumi Usui, Shinji Hayashi, Egon Gross, Joachim Diener, and Dmitri Kovalev,
"Generation of Singlet Oxygen at Room Temperature Mediated by Energy Transfer from Photo-excited Porous Si,”
Physical Review B, Vol. 70, 085311, pp. 1-5, (2004). |
| Photoluminescence (PL) from singlet oxygen generated by energy transfer
from porous Si is observed at room temperature in an organic solvent. The
evidence of the indirect excitation by energy transfer is obtained from
PL excitation spectroscopy. The excitation spectrum indicates that by using
porous Si as a photosensitizer, light of the entire visible range can be
utilized for singlet oxygen generation at room temperature. |
|
|
62. Minoru Fujii, Motofumi Usui, Shinji Hayashi, Egon Gross, Dmitri
Kovalev, Nicolai Künzner, Joachim Diener, and Victor Yu. Timoshenko,
"Chemical Reaction Mediated by Excited States of Si Nanocrystals -Singlet Oxygen Formation in Solution,”
Journal of Applied Physics, Vol. 95, No. 7, pp. 3689-3693, April (2004). |
| Formation of singlet oxygen in solution by using Si nanocrystals as photosensitizers
has been demonstrated. It has been shown that the absorption band of 1,3-diphenylisobenzofuran
(DPBF) in benzene centered at 416 nm decreases by irradiating green (514.5
nm) or red (632.8 nm) light if fresh porous Si powder is dispersed in the
solution. The decomposition of DPBF was observed only when fresh porous
Si was irradiated by light, i.e., without light irradiation no effects
were observed. Furthermore, the effect was drastically suppressed if porous
Si powder was annealed and a monolayer of oxide was formed on the surface
of nanocrystals. The rate of the decomposition of DPBF was accelerated
when the solution was bubbled by oxygen gas. These results indicate that
electronic excitation of Si nanocrystals is transferred to molecular oxygen
dissolved in solution, resulting in the formation of singlet oxygen. Generated
singlet oxygen reacts with DPBF (1,4-cycloaddition reaction), forming endoperoxides,
which in turn decompose to yield irreversible products. In addition to
the singlet-oxygen-mediated decomposition of DPBF, the possibility of direct
reaction between triplet excited states of Si nanocrystals and DPBF is
discussed. |
|
|
59. Egon Gross, Dmitri Kovalev, Nicolei Künzner, Joachim Diener, Frederick Koch, Vicotor Yu. Timoshenko, and Minoru Fujii,
"Spectrally Resolved Electronic Energy Transfer from Silicon
Nanocrystals to Molecular Oxygen Mediated by Direct Electron Exchange,”
Physical Review B, Vol. 68, 115405, pp. 1-11, (2003). |
| We report on a spectroscopic study of electronic energy transfer from excitons
confined in silicon nanocrystals to triplet ground-state oxygen molecules,
being either physisorbed on the nanocrystal surface or present in the gas
phase. The broad photoluminescence spectrum of the nanocrystal assembly
probes the transfer of excitation and verifies that nonresonant energy
transfer proceeds via multiphonon emission. At low temperatures a small
spatial separation of the interacting species and a long lifetime of triplet-state
excitons provide the strongest coupling. The energy-transfer time to the
first and second excited states of molecular oxygen is in the range of
100μs and shorter than 3μs, respectively. Nanocrystals with a chemically
modified surface are employed to demonstrate that energy transfer is governed
by direct electron exchange. Magneto-optical experiments reveal the importance
of the spin orientation of the exchanged electrons for the transfer rate.
In the regime of intermediate temperatures (110–250K) the transfer of excitation
to the O2 dimer is resolved. |
|
|
52. D. Kovalev, E. Gross, N. Künzner, F. Koch, V. Yu. Timoshenko, and M. Fujii,
"Resonant Electronic Energy Transfer from Excitons Confined in Silicon Nanocrystals to Oxygen Molecules,”
Physical Review Letters, Vol. 89, No. 13, pp. 137401-1-4, September (2002). |
| We demonstrate efficient resonant energy transfer from excitons confined in silicon nanocrystals to molecular oxygen (MO). Quenching of photoluminescence (PL) of silicon nanocrystals by MO physisorbed on their surface is found to be most efficient when the energy of excitons coincides with triplet-singlet splitting energy of oxygen molecules. The dependence of PL quenching efficiency on nanocrystal surface termination is consistent with short-range resonant electron exchange mechanism of energy transfer. A highly developed surface of silicon nanocrystal assemblies and a long radiative lifetime of excitons are favorable for achieving a high efficiency of this process. |
|
|
|
| |
| |

|
|
|

